BIG 2-13 / CL2-80881-001 (FINESSE)
ClinicalTrials.gov Identifier:NCT02053636
Sponsor
Servier
Key Collaborators
Breast International Group
Frontier Science Scotland
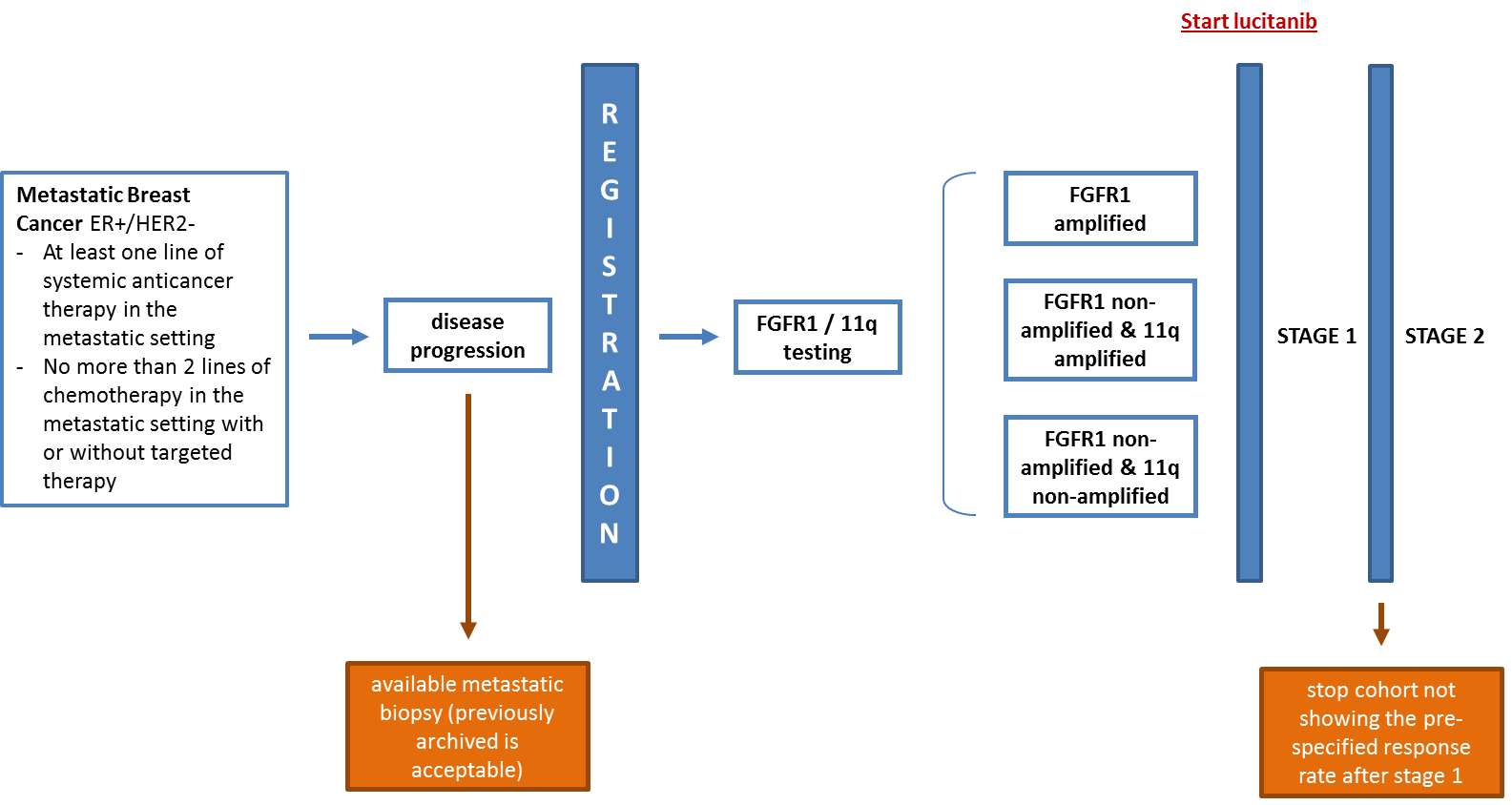
Aim
To evaluate the objective response rate (ORR) of single agent lucitanib in metastatic breast cancer patients with FGFR1-amplified, FGFR1-non amplified with 11q amplification, or FGFR1-non amplified without 11q amplification.
Added Value
This study is the first international trial in the metastatic setting to be carried out by the BrEAST Data Centre. This represents a new milestone for our organization. This trial is evaluating a FGFR inhibitor and determining if the benefit is restricted or not in patients according to FGFR amplification.
Recruitment
Recruitment started in December 2013 and the study is expected to complete in Q2/3 2016. Around 20-25 sites are participating in this trial.
Design