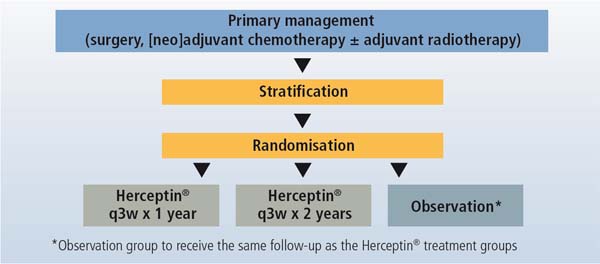
BIG 01-01 (HERA): Herceptin® adjuvant study
ClinicalTrials.gov Identifier:NCT00045032
Sponsor
Hoffmann-La Roche
Key Collaborators
Breast International Group
Frontier Science Scotland
Aim
To test whether the anti-HER2 therapy trastuzumab (Herceptin®) given for 1 or 2 years after standard chemotherapy improves disease-free and overall survival compared to observation in patients with over-expression of the HER-2 protein (HER-2 positive breast cancer).
Added Value
This landmark trial showed extraordinary results, with trastuzumab reducing by half the risk of relapse and by a third the risk of death from breast cancer in patients with HER-2 positive disease. Such positive results have not been reported for many years in oncology and have changed treatment practice worldwide for this patient population.
Recruitment
Recruitment started in December 2001 and was completed in June 2005. Drawing largely from the BIG network, the accrual rate exceeded all expectations, averaging over 200 patients per month.
In total, 5.102 patients were recruited in 39 countries at 482 sites. Over 20 research groups participated in the trial, 17 affiliated with BIG, 7 non-affiliated and some independent sites.
Outcomes
- The trial is in its follow-up phase. However, with the first pre-planned interim analysis of the data showing such an improvement in disease-free survival with adjuvant trastuzumab after 1 year of median follow-up, the results were published in the New England Journal of Medicine (2005) and, together with the combined analysis of two North American trials, were presented at the 2005 ASCO Annual Meeting.
- The second analysis at a median follow-up of 2 years showed an improvement in overall survival, confirming that trastuzumab is extremely efficacious. The results were published in the Lancet (2007).
- A third analysis at a median follow-up of 4 years showed continuous improvement in disease free survival but lost overall survival benefit, which was confounded by the high cross-over rate (around 50%) to the trastuzumab arm. In a non-randomized comparison, patients who selected to cross over to trastuzumab had improved DFS compared to those who remained in the observation arm. These results were published in Lancet Oncology (2011).
- The comparison between patients treated with 1 year or 2 years of trastuzumab showing no superior benefit of extending trastuzumab beyond one year. These results were published in Lancet (2013).
- Other publications have focused on other aspects of the study: - Cardiac safety data; Journal of Clinical Oncology (2007, 2010 and 2014). - Analyses of several subgroups showing the magnitude of benefit across all subgroups of patients; Annals of Oncology (2008). - Degree of HER-2 gene amplification and its impact on disease-free survival; Journal of Clinical Oncology (2009). - Outcome of women who became pregnant during or after exposure to trastuzumab; Breast Cancer Research and Treatment (2012). - Relationship between HER2 amplification and clinical outcome; Annals of Oncology (2013). - Detailed evaluation of patients who developed CNS metastasis; Lancet Oncol (2013). - Magnitude of benefit of trastuzumab in patients with HER2+ lobular carcinoma; Journal of Clinical Oncology (2013). - Efficacy of adjuvant trastuzumab in small tumors â¤2cm; J Clin Oncol 2015.
Tissue collection for translational research studies in association with the HERA trial (TransHERA)
The objectives of TransHERA are to collect, store and analyse breast cancer tissue and serum from patients included in the HERA trial to allow the identification of molecular characteristics that are associated with the differential likelihood of response or that allow detection of residual or relapsing disease. The research proposals selected by the TransHERA Committee to use this tissue are expected to help identify protein, genetic and molecular factors that might lead to new targets for the treatment of breast cancer or to a better understanding of the disease. This in turn can improve treatment strategies, enhance prevention and/or lead to cure.
Design